Alcohols, Phenols and Ethers Class 12 MCQs Questions with Answers
Question 1.
Among the following compounds, strongest acid is
(a) H-C = C-H
(b) C6H6
(c) C2H6
(d) CH3OH
Answer
Answer: (d) CH3OH
Question 2.
1-Propanol and 2-propanol can be best distinguished by
(a) Oxidation with KMnO4 followed by reaction with Fehling solution?
(b) Oxidation with acidic dichromate followed by reaction with Fehling solution.
(c) Oxidation by heating with copper followed by reaction with Fehling solution.
(d) Oxidation with cone. H2SO4 followed by reaction with Fehling solution.
Answer
Answer: (c) Oxidation by heating with copper followed by reaction with Fehling solution.
Question 3.
The compound which gives the most stable carbonium ion on dehydration is
(a) (CH3)2CHCH2OH
(b) (CH3)3COH
(c) CH3CH2CH2CH2OH
(d) CH3CH OH CH2 CH3
Answer
Answer: (b) (CH3)3COH
Question 4.
In the following compounds:

The order of acidity is
(a) III > IV > I > II
(b) I > IV > III > II
(c) II > I > III > IV
(d) IV > III > I > II
Answer
Answer: (d) IV > III > I > II
Question 5.
In CH3 CH2 OH, the bond that undergoes heterolytical change most readily is
(a) C-C
(b) C-O
(c) C-H
(d) O-H
Answer
Answer: (d) O-H
Question 6.
Phenol reacts with Br2 in CS2 at low temperature to give
(a) o-Bromophenol
(b) o-and p-promophenols
(c) p-Bromophenol
(d) 2, 4, 6Tribromophenol
Answer
Answer: (b) o-and p-promophenols
Question 7.
In the reaction of phenol with CHCl3 and aqueous NaOH at 343 K, the electrophile attacking the ring is:
(a) CHCl3
(b) CHCl2
(c) CCl2
(d) COCl2
Answer
Answer: (c) CCl2
Question 8.
Which of the following is most acidic?
(a) Phenol
(b) Benzyl alcohol
(c) m-chlorophenol
(d) cyclohexanol
Answer
Answer: (c) m-chlorophenol
Question 9.
The correct order of boiling points for primary (1°), Secondary (2°) and Tertiery (3°) alcohols is
(a) 1° > 2° > 3°
(b) 3° > 2° > 1°
(c) 2° > 1° > 3°
(d) 2° > 3° > 1°
Answer
Answer: (a) 1° > 2° > 3°
Question 10.
When Phenol is distilled with zinc dust, it gives
(a) Benzene
(b) Toluene
(c) Benzaldehyde
(d) Benzoic acid
Answer
Answer: (a) Benzene
Question 11.
In the following reaction

(a) ethane
(b) ethylene
(c) butane
(d) propane
Answer
Answer: (a) ethane
Question 12.
Which of the following cannot be made by using Williamson Synthesis:
(a) Methoxybenzene
(b) Benzyl p-nitrophenyl ether
(c) tert. butyl methyl ether
(d) Ditert. butyl ether
Answer
Answer: (d) Ditert. butyl ether
Question 13.
The I.U.P.A.C. name of the ether CH2 = CH-CH2O CH3 is
(a) Alkyl methyl ether
(b) l-Methoxy-2-propene
(c) 3-Methoxy-l-propene
(d) Vinyl dimethyl ether
Answer
Answer: (c) 3-Methoxy-l-propene
Question 14.
Dehydration of alcohol to ethers is catalysed by
(a) cone. H2SO4 at 413 K
(b) Hot NaOH
(c) Hot HBr
(d) Hot HNO3
Answer
Answer: (a) cone. H2S04 at 413 K
Question 15.
Ethers are
(a) Neutral
(b) Basic
(c) Acidic
(d) Amphoteric
Answer
Answer: (b) Basic
Question 16.
The ether

when treated with HI produces

Answer
Answer: (a)
Question 17.
Among the following, the one which reacts most readily with ethanol is
(a) p-Nitrobenzyl bromide
(b) p-Chlorobenzyl bromide
(c) p-methoxybenzyl bromide
(d) p-methyl benzyl bromide.
Answer
Answer: (c) p-methoxybenzyl bromide
Question 18.
The major product formed by the reaction:

(a) CH3.CHCH3.CH2OCH3
(b) CH3 CH (OCH3) CH2 CH3

Answer
Answer: (c)
Question 19.
tert-Butyl methyl ether on heating with HI gives a mixture of
(a) tert-Butyl alcohol and methyl iodide.
(b) tert-Butyl iodide and methanol
(c) Isobutylene and methyl iodide
(d) Isobutylene and methanol.
Answer
Answer: (b) tert-Butyl iodide and methanol
Question 20.

(a) C6H5OC2H5
(b) C2H5OC2H5
(c) C6H5OC6H5
(d) C6H5I
Answer
Answer: (b) C2H5OC2H5
Question 21.
An ether is more volatile than alcohol having the same molecular formula. This is due to
(a) intermolecular hydrogen bonding in alcohols.
(b) dipolar character of ethers
(c) alcohols, having resonance structures
(d) intermolecular hydrogen bonding in ethers.
Answer
Answer: (a) intermolecular hydrogen bonding in alcohols.
Question 22.

(a) CH3CH Br CH3
(b) CH3 CH2 CH2 Br Br
(c) CH2 = CH-Br
(d) Br CH = CH-CH3
Answer
Answer: (a) CH3CH Br CH3
Question 23.
Strength of acidity is in order:

(a) II > I > III > IV
(b) III > IV > I > II
(c) I > IV > III > n
(d) IV > III > I > II
Answer
Answer: (b) III > IV > I > II
Question 24.
Cyclohexene is best prepared from cyclohexanol by which of the following:
(a) cone. H3PO4
(b) Cone. HCl/ZnCl2
(c) Cone. HCl
(d) Cone. HBr
Answer
Answer: (a) cone. H3PO4
Question 25.
Wood spirit is known as acetone
(a) methanol
(b) ethanol
(c) acetone
(d) benzene
Answer
Answer: (a) methanol
Question 26.
Acetone reacts with Grignard reagent to form
(a) 3° alcohol
(b) 2° alcohol
(c) ether
(d) no reaction
Answer
Answer: (a) 3° alcohol
Question 27.
The product of acid catalysed hydration of 2-phenylpropene is
(a) 3-Phenyl-2-propanol
(b) 1-Phenyl-2-propanol
(c) 2-phenyl-2-propanol
(d) 2-phenyl-1-propanol.
Answer
Answer: (a) 3-Phenyl-2-propanol
Question 28.
Which of the following compounds is most acidic?

Answer
Answer: (a)
Question 29.
Acid-cataylysed hydration of alkenes except ethene leads to the formation of
(a) primary alcohol
(b) secondary or tertiary alcohol
(c) mixture of primary and secondary alcohols.
(d) mixture of secondary and tertiary alcohols.
Answer
Answer: (b) secondary or tertiary alcohol
Question 30.
During dehydration of alcohols to alkenes by heating with cone. H2SO4 the initial step is
(a) formation of an ester
(b) protonation of alcohol molecule
(c) formation of carbocation
(d) elimination of water
Answer
Answer: (b) protonation of alcohol molecule



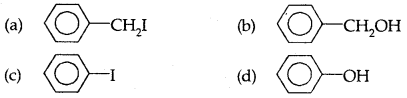














Comments
Post a Comment