The Solid State Class 12 MCQs Questions with Answers
Question 1.
Which of the following transition metal oxides is paramagnetic?
(a) TiO
(b) VO
(c) Cu2O
(d) Mn2O3
Answer
Answer: (b) VO
Question 2.
Which of the following transition metal oxides is not an insulator?
(a) MnO
(b) NiO
(c) VO
(d) Mn2O3
Answer
Answer: (c) VO
Question 3.
Coordination number in ccp and hep arrangements of metal atoms are respectively.
(a) 6, 6
(b) 12, 6
(c) 8, 6
(d) 12, 12
Answer
Answer: (d) 12, 12
Question 4.
An example of body centered cube is
(a) Sodium
(b) Magnesium
(c) Zinc
(d) Copper
Answer
Answer: (a) Sodium
Question 5
Fe3O4 is ferrimagnetic at room temperature but at 850 K it becomes
(a) Diamagnetic
(b) Ferromagnetic
(c) Non-magnetic
(d) Paramagnetic
Answer
Answer: (d) Paramagnetic
Question 6.
Which of the following is not an example of 13-15 compounds,
(a) InSb
(b) GaAs
(c) CdSe
(d) Alp
Answer
Answer: (c) CdSe
Question 7.
For tetrahedral coordination, the radius ration (r+/r ) should be
(a) 0.155-0.225
(b) 0.225-0.414
(c) 0.414-0.732
(d) 0.732-1
Answer
Answer: (b) 0.225-0.414
Question 8.
A compound formed by elements A and B crystallises in the cubic structure where A atoms are at comers of a cube and B atoms are at face centres. The formula of the compound is
(a) AB3
(b) A2B
(c) AB2
(d) A2B3
Answer
Answer: (a) AB3
Question 9.
How many space lattices are possible in a crystal?
(a) 23
(b) 7
(c) 230
(d) 14
Answer
Answer: (d) 14
Question 10.
The number of atoms in bcc arrangement is
(a) 1
(b) 2
(c) 4
(d) 6
Answer
Answer: (b) 2
Question 11.
In face-centered cubic unit cell, edge length is
(a) 4√3r
(b) 4√2r
(c) 2r
(d) √32r
Answer
Answer: (b) 4√2r
Question 12.
Metallic lustre is explained by
(a) diffusion of metal ions
(b) oscillation of loose electrons
(c) excitation of free protons
(d) existence of bcc lattice.
Answer
Answer: (b) oscillation of loose electrons
Question 13.
In a face-centered cubic lattice, unit cell is shared equally by how many unit cells?
(a) 4
(b) 2
(c) 6
(d) 8
Answer
Answer: (c) 6
Question 14.
The percentage of iron present as Fe (III) in Fe0.93 O1.0 is
(a) 17.7%
(b) 7.84%
(c) 11.5%
(d) 9.6%
Answer
Answer: (b) 7.84%
Question 15.
Which of the following fee structure contains cations in alternate tetrahedral voids? (IIT 2005)
(a) NaCl
(b) ZnS
(c) Na2O
(d) CaF2
Answer
Answer: (b) ZnS
Question 16.
The numbers of tetrahedral voids in the unit cell of a face centered cubic lattice of similar atoms is
(a) 4
(b) 6
(c) 8
(d) 10
(e) 12
Answer
Answer: (c) 8
Question 17.
The hardest substance among the following is
(a) Be2C
(b) Graphite
(c) Titanium
(d) SiC
(e) B4C
Answer
Answer: (d) SiC
Question 18.
How many unit cells are present in a cube-shaped ideal crystal of NaCl of mass 1.00 g? (Atomic mass of Na = 213, Cl = 35.5)
(a) 5.14 × 1021
(b) 1.28 × 1021
(c) 1.71 × 1021
(d) 2.57 × 1021
Answer
Answer: (d) 2.57 × 1021
Question 19.
What is the coordination number of Cl- in NaCl crystals
(a) 8
(b) 6
(c) 4
(d) 3
Answer
Answer: (b) 6
Question 20.
The pyknometric density of sodium chloride crystal is 2.165 × 10³ kg m-3, while its X-ray density is 2.178 × 10³ kg m-3. The fraction of the unoccupied sites in sodium chloride crystal is
(a) 5.96
(b) 15.96 × 10-2
(c) 5.96 × 10-1
(d) 5.96 × 10-3
Answer
Answer: (d) 5.96 × 10-3
Question 21.
If Z is the number of atoms in the unit cell that represents the closest packing sequence….ABC ABC….the number of tetrahedral voids in the unit cell is equal to
(a) Z
(b) 2Z
(c) Z/2
(d) Z/4
Answer
Answer: (b) 2Z
Question 22.
Which has no rotation of symmetry?
(a) Hexagonal
(b) Orthorhombic
(c) Cubic
(d) Triclinic
Answer
Answer: (d) Triclinic
Question 23.
If the distance between Na+ and Cl– ions in sodium chloride crystal is X pm the length of the edge of the unit cell is
(a) 4X pm
(b) X/4 pm
(c) X/2 pm
(d) 2 × pm
Answer
Answer: (d) 2 × pm
Question 24.
Which of the following metal oxides is antiferromagnetic in nature?
(a) MnO2
(b) TiO2
(c) VO2
(d) CrO2
Answer
Answer: (d) CrO2
Question 25.
The interionic distance for cesium chloride crystal will be
(a) a
(b) a/2
(c) √3 a/2
(d) 2a/√3
Answer
Answer: (c) √3 a/2
Question 26.
The liquified metal expanding on solidification is
(a) Ga
(b) Al
(c) Zn
(d) Cu
Answer
Answer: (a) Ga
Question 27.
The number of unit cells in 58.5 g of NaCl is nearly
(a) 6 × 1020
(b) 3 × 1022
(c) 1.5 × 1023
(d) 0.5 × 1024
Answer
Answer: (d) 0.5 × 1024
Question 28.
The number of octahedral sites per sphere in fee structure is
(a) 8
(b) 4
(c) 2
(d) 1
Answer
Answer: (d) 1
Question 29.
The crystal system of a compound with unit cell dimensions a = 0.387, b = 0.387 and c – 0.504 nm and α = ß = 90° and γ = 120° is
(a) Cubic
(b) Hexagonal
(c) Orthorhombic
(d) rhombohedral
Answer
Answer: (b) Hexagonal
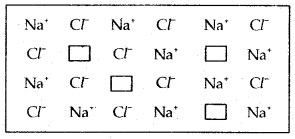
Question 30.
Which type of crystal defect is indicated in the diagram below?

(a) Frenkel defect
(b) Schottky defect
(c) Interstitial defect
(d) Frenkel and Schottky defects.
Answer
Answer: (b) Schottky defect








Comments
Post a Comment